Report Materials
Why OIG Did This Audit
The Government Charge Card Abuse Prevention Act of 2012 (Charge Card Act), P.L. No. 112-194, requires Offices of Inspectors General (OIGs) to conduct annual risk assessments of agency travel card programs. OIGs must report to the Office of Management and Budget (OMB) on the results of their analyses by January 31 of each year.
We used the risk areas of the Committee of Sponsoring Organizations of the Treadway Commission's (COSO) Enterprise Risk Management-Integrated Framework and OMB Compliance Standards to assess the Food and Drug Administration's (FDA) ability to manage internal controls and risk in its travel card program.
Our objective was to analyze the risk of illegal, improper, or erroneous purchases in the FDA travel card program and to determine whether FDA has designed and implemented controls and strategies to mitigate these potential risks.
How OIG Did This Audit
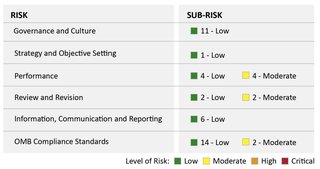
We interviewed FDA management, performed travel transactions testing, reviewed documents, and evaluated FDA's responses to an OIG questionnaire. Based on this review, we used the COSO framework and the OMB Compliance Standards to identify 6 risk areas and 46 sub-risk areas.
What OIG Found
FDA generally designed and implemented controls and strategies to mitigate the potential risks of illegal, improper, or erroneous purchases in its travel card program. Within the 6 risk areas related to the FDA travel card program, we identified 46 sub-risk areas and assessed 38 as low risk and 8 as moderate risk. Overall, we assessed the FDA travel card program as low risk.
What OIG Recommends
This report contains no recommendations.
Notice
This report may be subject to section 5274 of the National Defense Authorization Act Fiscal Year 2023, 117 Pub. L. 263.