Report Materials
WHY WE DID THIS STUDY
A 2018 report by the Medicare Payment Advisory Commission found that Medicare paid substantially more than commercial payers for certain items, including intermittent urinary catheters. The report recommended that Medicare incorporate such items into its competitive bidding program, thereby reducing the rates that Medicare allows. However, Medicare has not done so. In fiscal year (FY) 2020, Medicare Part B and its beneficiaries paid $407 million for all intermittent urinary catheters. OIG evaluated whether Medicare's payment amounts for these catheters may offer the potential for savings.
HOW WE DID THIS STUDY
We sampled 600 Medicare claims from FY 2020, for the three billing categories of intermittent urinary catheters. We requested that suppliers provide and document the acquisition cost for each of the catheters in these claims. We compared the suppliers' acquisition costs to Medicare payment amounts in three ways: in total, across the three categories, and with regard to three specific features. Additionally, we interviewed CMS to better understand the methods available to reduce payment rates while maintaining beneficiaries' access to the catheters that best serve their medical needs.
WHAT WE FOUND
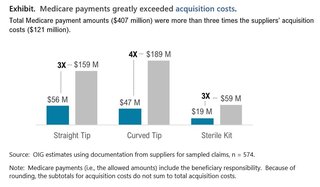
From our analysis of data submitted by suppliers, we estimated that Medicare payments were 3.4 times suppliers' acquisition costs for intermittent urinary catheters in FY 2020. In total, Medicare allowed $407 million in payments for these items, while suppliers paid approximately $121 million to acquire them (see exhibit below). Medicare payments exceeded suppliers' acquisition costs by $286 million. Each of the three billing categories of intermittent urinary catheters (straight tip, curved tip, and sterile kit) showed large differences between Medicare payments and acquisition costs, which indicates a potential for substantial savings both to Medicare and beneficiaries, who share responsibility for paying the Medicare-allowed amount.
We found potential savings across all categories of catheters. We also found potential savings when catheters had one or more of three specific features (hydrophilic coating, grip, or sleeve)-in these cases, there were smaller but still meaningful differences between Medicare payment rates and supplier acquisition costs, reinforcing the potential for savings.
We recognize that suppliers face other costs beyond the cost of acquiring catheters and need an opportunity to maintain a profit. However, the magnitude of the differences between Medicare reimbursements and suppliers' acquisition costs indicates that Medicare and its beneficiaries can achieve substantial savings while allowing for other costs. To provide an example of the potential for savings, we performed an illustrative analysis of suppliers' other costs. In this analysis, we used data in a report from the home health care industry (not specific to catheters), which estimated that for every dollar spent on acquisition costs, suppliers spent an additional 72 cents in other costs. We applied this same proportion of other costs to our data on acquisition costs in order to obtain an example of suppliers' total costs. This illustrative analysis yields a total cost of $209 million, which would allow $198 million in potential Medicare savings and supplier profits. We believe that this analysis likely underestimates potential savings by overstating suppliers' other costs.
WHAT WE RECOMMEND
We recommend that CMS lower Medicare's payment rates for intermittent urinary catheters. As it does so, CMS should continue to take steps to ensure beneficiaries' access to the catheters that best serve their medical needs. When CMS has previously sought to obtain savings for other items, it has used competitive bidding or its "inherent reasonableness" process. Each of these mechanisms has its own methods for ensuring beneficiary access. CMS did not explicitly indicate whether it concurred with the recommendation; instead, CMS stated that it will take our recommendation under consideration as it determines appropriate next steps.
View in Recommendation Tracker
Notice
This report may be subject to section 5274 of the National Defense Authorization Act Fiscal Year 2023, 117 Pub. L. 263.